Orange County Based Company Focuses on Innovative Solutions for Neurovascular Diseases
MicroVention was founded and incorporated in a small facility in 1997 in Aliso Viejo, California. From the beginning, it was our founding principle to establish the strategic objective of developing medical devices, which will enable or significantly improve the treatment of diseases of small blood vessels in the brain.
After developing early concepts and prototype devices for the treatment of acute embolic stroke and embolization of cerebral aneurysms, our company narrowed its focus to aneurysm therapy, developing a line of implantable, detachable neuroendovascular platinum coils. From these early concepts, our first products were born: The MicroPlex® Coil System, a bare platinum coil, and the HydroCoil® Embolic System, a polymer gel-coated platinum coil. These products have evolved over the last 20 years and we continue to expand those product lines with new state-of-the-art technologies for the treatment of cerebral aneurysms.
In 2006, MicroVention was acquired by the Japan-based TERUMO® Corporation which was established in 1921. With TERUMO’s support, MicroVention has continued to operate autonomously, which has allowed the company to grow independently and more rapidly. From our modest beginnings, we have experienced significant growth, capacity, and capability.
Today, MicroVention’s extensive intellectual property and a strong R&D pipeline support ongoing innovation in neurointerventional devices and a continuously expanding product portfolio that includes over 30 devices. We are a global company with focus on disease-based solutions: Aneurysm Therapy Solutions, Ischemic Stroke and Carotid Artery Solutions, Neurovascular Malformation Solutions, and a comprehensive line of Access Products. The company is comprised of approximately 3,000 associates worldwide with a reach of over 75+ countries.

“MicroVention’s success relies heavily on the partnership with our customers.”
– RICH CAPPETTA, President and CEO
In 2016, MicroVention recently expanded into its new worldwide Innovation Center in in Aliso Viejo, California. Our new building provides technological and environmental efficiency advantages, a larger manufacturing area, and expansive research and development labs. As MicroVention continues to expand, we are implementing a comprehensive strategic agenda focused on becoming the #1 global leader in neuroendovascular therapy in just a few short years.
In 2016, MicroVention recently expanded into its new worldwide Innovation Center in in Aliso Viejo, California. Our new building provides technological and environmental efficiency advantages, a larger manufacturing area, and expansive research and development labs. As MicroVention continues to expand, we are implementing a comprehensive strategic agenda focused on becoming the #1 global leader in neuroendovascular therapy in just a few short years.

MicroVention, Inc. Worldwide Headquarters in Aliso Viejo, California.
In 2018, we were proud to offer of our 2 most recent innovations, both obtained by PMA approval, the most rigorous standard of FDA approval, to the neuroendovascular market in the United States with the introduction of the WEB™ System and the LVIS® and LVIS® Jr. stents.
The WEB® System is a unique, single-device treatment solution for wide neck bifurcation aneurysms, which may account for up to 35% of all aneurysms. When placed inside the aneurysm sac, the WEB® device’s proprietary microbraid technology bridges the aneurysm neck, disrupting blood flow, and creates a scaffold for long-lasting treatment.
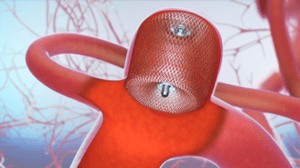
WEB® System placed inside the aneurysm sac to disrupt blood flow.
The LVIS ®and LVIS® Jr. stents are the first and only stents PMA approved for stent-assisted coil embolization and only the second PMA approved device designed for intracranial aneurysm treatment. The LVIS® and LVIS® Jr. stents (Low profileVisualizedIntraluminal Support) feature a braided conformable, resheathable and retrievable design that provides high metal coverage and end-to-end device visualization to provide support for even the smallest neurovascular embolization coils for the treatment of wide-necked saccular intracranial aneurysms.
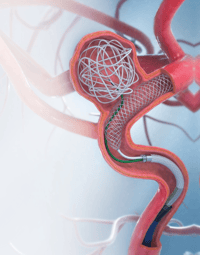
The coils, in conjunction with the LVIS® stent prevents blood from flowing into the aneurysm and allow the aneurysm to occlude.
Looking toward the future, we are committed to excellence in everything we do, and we strive to continually improve and innovate. We will continue to achieve results that exceed expectations—our own and our customers’.


Let Us Know What You Thought about this Post.
Put your Comment Below.